Description
The endometrium has an extraordinary regenerative capacity, mainly controlled by endometrial stem cells that reside in its basal layer. However, other sources of stem cells, such as the bone marrow, also contribute to maintaining the natural balance (homeostasis) of this tissue.
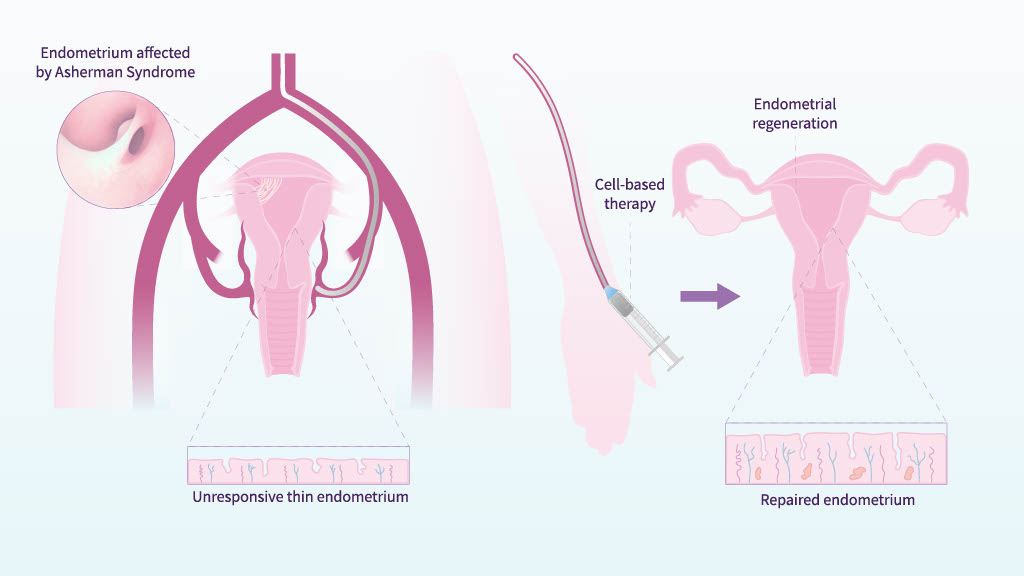
When the stem cell niche is damaged, healthy endometrium is replaced by fibrotic tissue, leading to endometrial atrophy or Asherman’s Syndrome, conditions often considered incurable.
Our group has demonstrated the therapeutic potential of autologous bone marrow–derived CD133+ stem cells to restore endometrial function in patients affected by these pathologies.
To date, we have completed a proof of concept and a Phase II clinical trial, which have not only confirmed the safety of this therapy, but also provided deeper insights into its mechanisms of action, clinical effects, and its impact on endometrial regeneration.
We are currently working on the design of a Phase III study, which we plan to initiate in the near future. Altogether, these advances are bringing us closer to developing specific, targeted therapies capable of effectively treating a disease that has long been considered incurable.
Description
The endometrium has an extraordinary regenerative capacity, mainly controlled by endometrial stem cells that reside in its basal layer. However, other sources of stem cells, such as the bone marrow, also contribute to maintaining the natural balance (homeostasis) of this tissue.
When the stem cell niche is damaged, healthy endometrium is replaced by fibrotic tissue, leading to endometrial atrophy or Asherman’s Syndrome, conditions often considered incurable.
Our group has demonstrated the therapeutic potential of autologous bone marrow–derived CD133+ stem cells to restore endometrial function in patients affected by these pathologies.
To date, we have completed a proof of concept and a Phase II clinical trial, which have not only confirmed the safety of this therapy, but also provided deeper insights into its mechanisms of action, clinical effects, and its impact on endometrial regeneration.
We are currently working on the design of a Phase III study, which we plan to initiate in the near future. Altogether, these advances are bringing us closer to developing specific, targeted therapies capable of effectively treating a disease that has long been considered incurable.

Team members

Xavier Santamaria M.D., Ph.D.

Maria Pardo, Ph.D.

Estefania Fernandez

Javier Gonzalez, Ph.D.

Jose Serrano

Michael Erik Robles
Main Publications
Decoding the endometrial niche of Asherman’s Syndrome at single-cell resolution
Santamaria, X., Roson, B., Perez-Moraga, R. et al. Decoding the endometrial niche of Asherman’s Syndrome at single-cell resolution. Nat Commun 14, 5890 (2023). https://doi.org/10.1038/s41467-023-41656-1
Uterine stem cells: from basic research to advanced cell therapies
Santamaria X, Mas A, Cervello I, Taylor H, Simon C. Hum Reprod Update. 2018 Nov 1;24(6):673-693. https://doi.org/10.1093/humupd/dmy028. PMID: 30239705
Leucine-rich repeat-containing G-protein-coupled receptor 5-positive cells in the endometrial stem cell niche
Cervello I, Gil-Sanchis C, Santamaria X, Faus A, Vallve-Juanico J, Diaz-Gimeno P, Genolet O, Pellicer A, Simon C. FERTIL STERIL. 2017 Feb;107(2):510-519.e3. https://doi.org/10.1016/j.fertnstert.2016.10.021. Epub 2016 Nov 22.PMID: 27887719
Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: a pilot cohort study
Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, Ferro J, Palmero J, Remohi J, Pellicer A, Simon C.
Hum Reprod 2016; 31(5):1087-96. https://doi.org/10.1093/humrep/dew042
Human CDD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome
Cervello I, Santamaria X, Gil-Sanchis C, Faus A, Cabanillas S, Simon C. FERTIL STERIL 2015; 104(6):1552-60.E1-3. https://doi.org/10.1016/j.fertnstert.2015.08.032
