The endometrium is a tissue with an extraordinary regenerative capacity. After each menstrual cycle, it undergoes complete renewal to prepare the uterus for potential embryo implantation. This process, which is essential for fertility and women’s reproductive health, reflects the strong regenerative potential of the human endometrium and its tight regulation by ovarian hormones.
Building on this foundation, the Progenitor Project, also referred to as EndoProg, addresses a central question in reproductive biology: which cells enable the cyclical regeneration of the human endometrium.
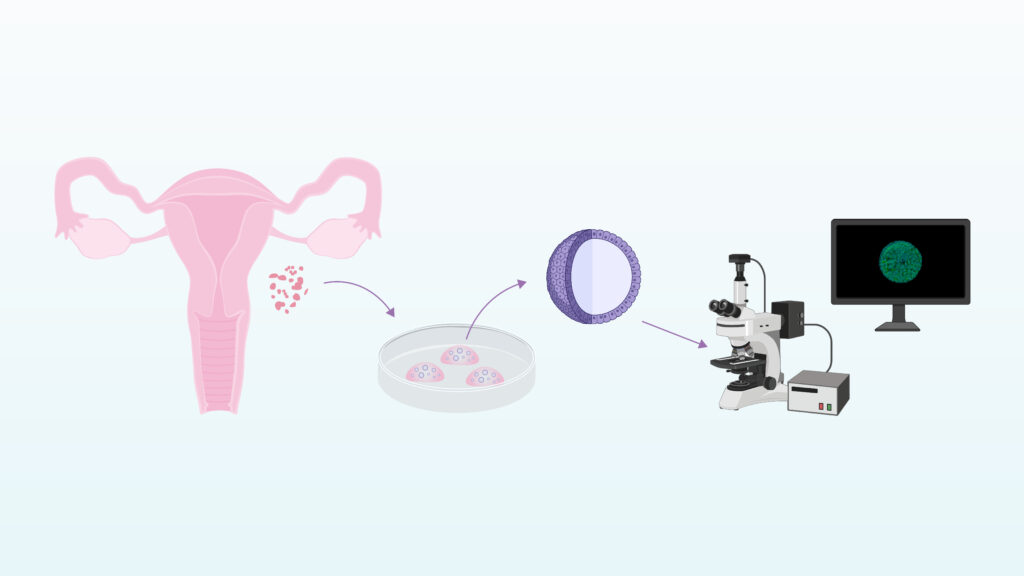
To explore this, the research team analyzed human endometrial biopsies at single-cell resolution. This approach makes it possible to examine gene expression in individual cells, determine their cellular identity, such as epithelial, stromal, or immune cells, and identify genes that are differentially expressed within each population.
Through this analysis, a group of cells expressing genes associated with adult stem cell features was identified. Among them, SOX9 stands out as a marker closely linked to self-renewal and tissue regeneration. Following a detailed assessment of the different endometrial cell types, two candidate cell populations were selected as potential drivers of endometrial regeneration. This work also enabled the identification of specific cell surface markers to isolate these populations and culture them under controlled conditions.
The next step of the project is essential to validate these findings. The candidate cell populations are being isolated and cultured as endometrial organoids, an advanced experimental model that reproduces key aspects of human endometrial architecture and function in vitro and has become a central tool for the study of endometrial biology under physiological and pathological conditions.
In regenerative tissues such as the endometrium, organoids arise from adult stem cells. Evaluating whether these cells generate organoids, and how they organize and develop, will help determine whether they truly possess regenerative capacity and clarify their role in cyclical endometrial renewal.
In the medium term, this research will improve our understanding of how endometrial regeneration is regulated and how ovarian hormones contribute to this process. From a clinical perspective, it lays the groundwork for future therapeutic strategies targeting conditions in which endometrial regenerative capacity is impaired, such as Asherman syndrome or thin endometrium. Identifying the cells that drive endometrial regeneration is a critical step toward restoring endometrial function when this process fails.
