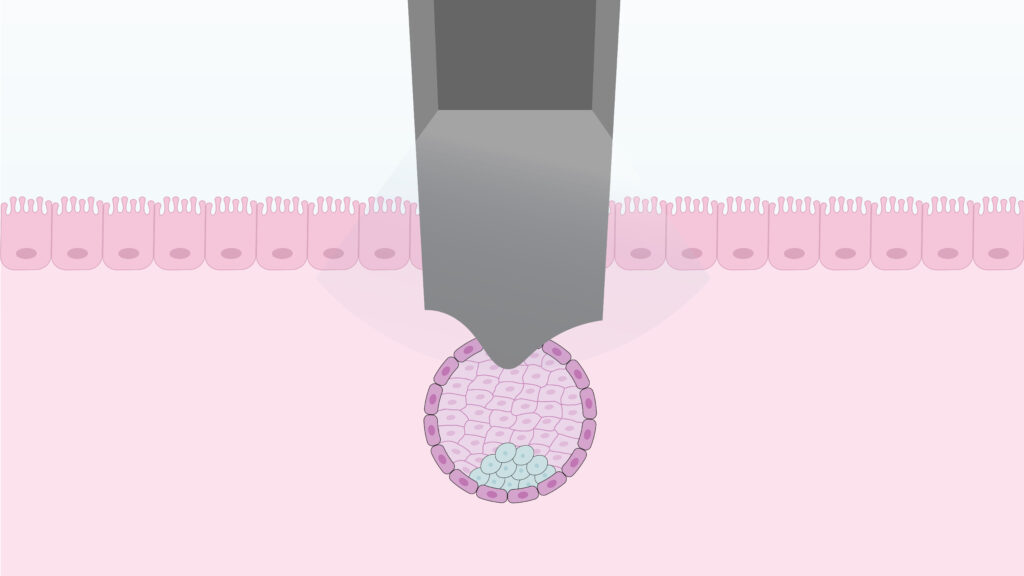
For more than four decades, embryo transfer has remained essentially unchanged. Despite major advances in embryo culture and morphological assessment, the final and most decisive step still relies on a manual procedure guided by ultrasound and individual expertise. A variation of just a few millimeters in depth or placement can shape the entire trajectory of a pregnancy. Once the embryo crosses the endometrial surface, everything becomes invisible. No imaging technology allows us to see how it adheres, invades, or how the maternal tissue responds. We only know the outcome days later, while the defining biological events remain hidden inside the uterus.
Recent progress in micro-engineering, controlled perfusion systems, and 3D tissue models is transforming this landscape. Embryo transfer can now be approached not as a manual placement, but as a precise, standardized, and measurable biological event. Instead of relying on subjective positioning, it becomes possible to deliver an embryo into a receptive niche with controlled spatial and mechanical conditions that influence the earliest steps of implantation.
From manual placement to controlled perfusion
At the Carlos Simon Foundation and Premium Fertility, we have developed the TD System (Transfer Direct) to redefine this critical moment. The TD System performs an automated, direct implantation by placing the embryo exactly where early invasion is expected to occur, while minimizing manipulation that could alter its developmental behavior.
This approach draws on principles similar to perfusion-based systems used in organ preservation and tissue engineering. By controlling variables that were previously immeasurable, TD transforms embryo transfer from a manual act into a reproducible, quantifiable procedure that can be studied experimentally.
INVADE: observing immediate post-implantation events
To validate this new form of implantation ex vivo, we created INVADE, a 3D in vitro platform designed to visualize the earliest interactions between the embryo and its surrounding matrix after an automated TD transfer.
Using INVADE, mouse and human embryos remained viable for up to three days following TD-based deposition. During this period, they demonstrated:
- Localized remodeling of the surrounding gel by trophectoderm cells
- Detectable secretion of hCG, indicating active endocrine signaling
- Early embryo–matrix interactions comparable to the first stages of implantation
For the first time, these observations provide a direct experimental window into post-implantation dynamics, offering insights that cannot be obtained in vivo.
A new perspective on the maternal–fetal interface
Together, TD System and INVADE introduce a new framework for studying implantation and early pregnancy. Instead of inferring processes from clinical outcomes, we can now observe and quantify them under controlled conditions.
This engineered approach to implantation opens the door to understanding how the embryo communicates with the endometrium, how maternal tissues respond, and which mechanical or biochemical cues may optimize success. It also provides a foundation for testing new therapies, designing more accurate diagnostic tools, and improving reproductive outcomes.
Ultimately, this shift allows us to study a biological space that has always been essential yet inaccessible: the moment when embryo and endometrium first meet.
